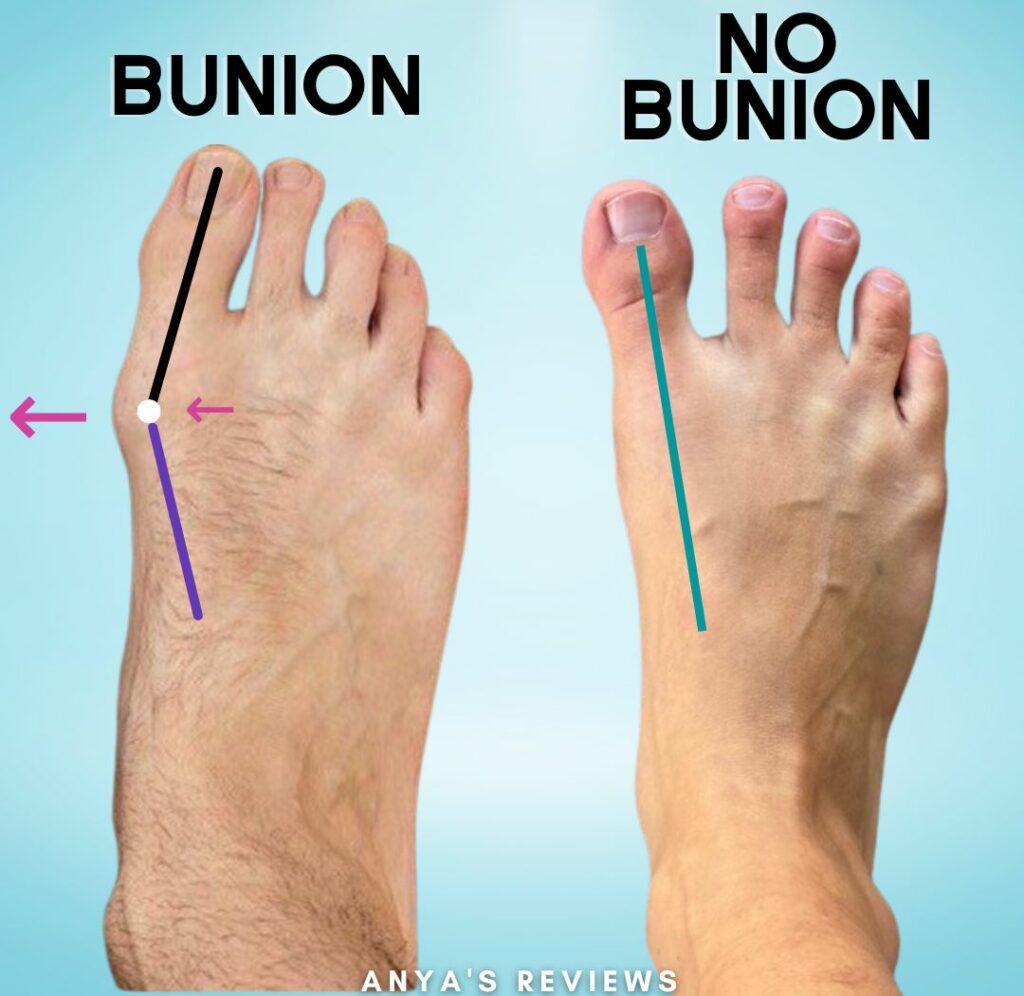
Are you in need of a bunionectomy? Consider participating in our clinical research study on an investigational non-opioid medication for pain relief. This Phase 2 study is designed to evaluate the safety and efficacy of VX-993. Participants will be required to stay on-site for 48 hours after surgery and will be compensated for study related efforts.
CONTACT US ABOUT BUNIONECTOMY TRIALBUNIONECTOMY
Description
Eligibility Criteria
Adults aged 18-75, Scheduled for bunion surgery
Intervention
Participants in this trial will receive one of the following treatments: VX-993: An investigational pain relief medication in three different dosages, Hydrocodone/Acetaminophen (HB/APAP), or placebo.
Duration
This study requires a 3-day commitment, which includes: day one, surgery and treatment initiation, followed by a 48-hour stay post-surgery for monitoring. Then a follow-up visit 14 days after surgery to assess your progress and any effects from the study medication.